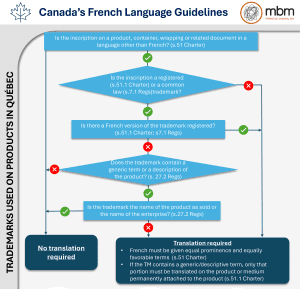
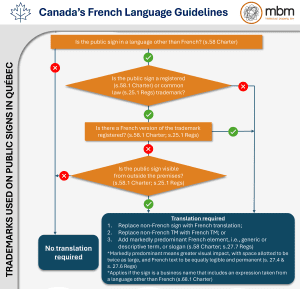
In the academic world, it is common for researchers to attend and present their findings at conferences. Papers presented will typically end up as part of conference handouts or available online for future use. PowerPoint slides used in presentations are also sometimes published or distributed. Posters are often set up where attendees may view them. Conversations between researchers happen after presentations, and at numerous coffee breaks and networking events. In the publish or perish world of academia, conferences provide one of the best venues to present your ideas and research and meet with like-minded people.
Similarly, in the corporate world tradeshows are often used to show off products and demonstrate their features and capabilities to potential customers. Trade show booths often include demonstration systems, brochures, and marketing presentations that are only available for the two or three days of the show. Copies of brochures and presentations may be saved or may be destroyed afterwards.
Unfortunately, what is good for the sharing of information is often not good for the patenting of inventions that arise from the research and products presented at these conferences. The subject matter of a patent claim must not have been previously disclosed, and the invention must not be obvious to a person skilled in the art or science to which it pertains[1]. Conference presentations, presentation slides, and posters can all be prior art, whether they come from an inventor or someone else, and can prevent you from patenting your inventions.
Posters are an interesting case in that they may often be displayed for just a few hours, be viewed by passersby, and usually do not become part of the published conference proceedings. They are often untraceable or destroyed later. They often will not contain enough information to prevent an invention from being novel, but nevertheless may form part of the state of the art that must be considered when determining if an invention contains an inventive step.
In Biogen Canada Inc. v. Taro Pharmaceuticals Inc., 2020 FC 621, a poster was presented at a conference in Baltimore in 2002, 18 years previous. The poster was available to the court but had only been presented for a short time at the conference, and in the intervening years could not have been found even with a reasonably diligent search. Nevertheless, expert testimony established that the poster was indeed genuine and therefore its contents formed part of the state of the art in 2002 for determining obviousness of the patent claims in question. The poster, together with information found in other sources of prior art, were enough to find the patent claims in question obvious and invalid. This case is interesting since a poster, only presented for a short time at the conference and thereafter not being available, was used to establish the state of the prior art 18 years ago.
In Mediatube Corp. v. Bell Canada, 2017 FC 6 the plaintiff alleged that the defendant’s Fibe TV service could be modified to infringe its patents. Bell argued that all limitations of the relevant patent claims had been disclosed in a number of sources, including brochures and prototype systems that had been presented at the SuperComm tradeshow in June 1998, 19 years previous. Brochures were available to the court. Mediatube argued that the brochure was only disclosed at the tradeshow and could not be considered to have been available to the public as it could not later be found in a reasonably diligent search by a skilled person. With the help of expert testimony, the court decided that the brochures and presentations of the systems, despite only being available for a short time, were part of the state of the art at the time and could be considered when determining the validity of Mediatube’s patent claims.
On the other hand, in Valence Technology, Inc. v. Phostech Lithium Inc., 2011 FC 174, the defendant was challenging the validity of plaintiff’s patents. Phostech asserted that conference publications, presentations, and posters presented twelve years previous were prior art to at least some of the patent claims. The presenter had also had discussions while at the conference. The poster had since been destroyed and could not be presented to the court. When defining the common general knowledge at the key date the judge decided to exclude the presentations, posters, and any discussions that may have happened. Though not stated, this may have been because the poster had been destroyed and that there were no experts to testify to its contents or importance.
It is difficult to determine in advance if a poster will later be found to form part of the state of the art when considering patent validity. For prior art, such as a poster, that may only be presented for a few hours at a scientific or industry conference, it is uncertain whether it can be considered part of the body of prior art of which a person skilled in the art could be said to possess, especially since it may not be found later even through a reasonably diligent search. Like any disclosure, the best practice is to:
1. Review all public disclosures, even those that are short-lived and will be unavailable later.
2. Be careful what kind of information you put on a poster. Try to use more general information on all conference materials if possible.
3. If you must disclose detailed information, restrict any disclosures to individuals or groups in a non-public space under NDA.
4. File a provisional or utility patent application before the event.
5. Review and document what others present. Smartphones make it easy.
Better to be safe than sorry!
If you are considering filing a patent and are worried about your conference/event disclosure, please feel free to contact MBM for a free consultation.
This article is general information only and is not to be taken as legal or professional advice. This article does not create a solicitor-client relationship between you and MBM Intellectual Property Law LLP. If you would like more information about intellectual property, please feel free to reach out to MBM for a free consultation.